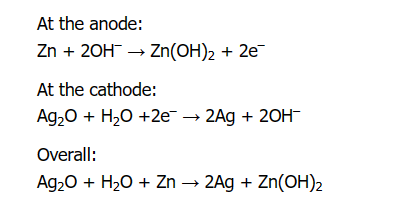on April 24th
715
Silver Oxide Batteries and Alkaline Batteries: Working Principle, Characteristics and Differences
Silver oxide and alkaline batteries, exemplified by the SR626SW and LR626 models respectively, serve critical roles in modern electronic applications, from precision timekeeping to powering various portable devices. Understanding the fundamental distinctions and operational mechanics between these battery types not only informs user choice but also highlights the technological innovations that have refined battery performance over decades. Silver oxide batteries utilize a combination of zinc and silver oxide to create a dependable source of power through well-defined electrochemical reactions. This process not only generates a stable output voltage but also exemplifies the efficiency of using silver oxide in battery technology. Conversely, alkaline batteries, typified by the LR626 model, rely on the interaction between zinc and manganese dioxide, facilitated by an alkaline electrolyte, to supply power. While they are more economically produced and widely used for a variety of everyday electronic devices, their rapid voltage decline can be a drawback in devices requiring consistent voltage levels. This comparative analysis not only underscores the different suitable applications based on battery characteristics but also emphasizes the need for consumers to choose based on the specific energy requirements and operational stability of their devices.
Catalog
Figure 1: Comparison between Silver Oxide Battery and Alkaline Battery
Definition
Silver oxide batteries are a specific type of primary battery that uses zinc as the anode and silver oxide as the cathode to generate an electric current through electrochemical reactions. These batteries are compact and have a high energy density, making them ideal for devices that require small size and consistent, stable voltage. The development of silver oxide batteries dates back to the 1930s, pioneered by Andre, building on the zinc/silver cell technology first demonstrated by Volta in the 19th century.
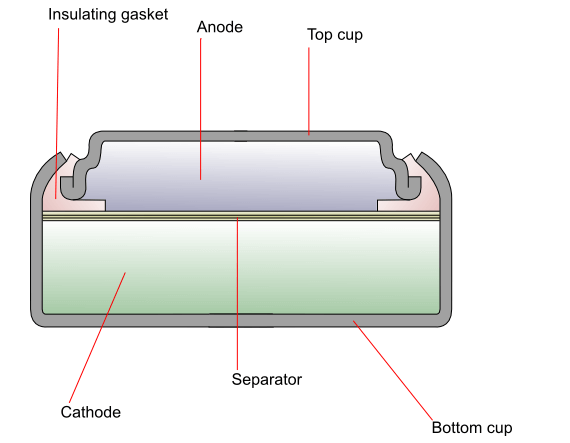
Figure 2: Silver Oxide Battery Internal Diagram
Work Principle
In a silver oxide battery, the zinc anode easily oxidizes from Zn(0) to Zn(II), releasing electrons in the process. The stability provided by the filled d-orbitals in the Zn(II) state makes zinc an excellent candidate for the anode material. At the cathode, these electrons reduce silver oxide to metallic silver while generating hydroxide ions as byproducts, helping to maintain the chemical balance within the electrolyte.
The electrochemical reactions in a silver oxide battery unfold as follows: Zinc reacts with hydroxide ions at the anode to produce zinc hydroxide and electrons Zn + 2OH- → ZnO + H2O+2e-. These electrons travel through the external circuit to the cathode where they react with silver oxide and water to produce silver and more hydroxide ions (Ag2O + 2e- + H2O → 2Ag + 2OH-). The overall battery reaction, Ag2O + Zn + H2O → 2Ag + Zn(OH)2, results in an open-circuit voltage of about 1.55 volts, indicating a high energy output.
Figure 3: Silver Oxide Battery Reaction Chemical Formula
Battery Characteristics
Silver oxide batteries are also designed with unique features such as using highly alkaline electrolytes, typically sodium hydroxide or potassium hydroxide. These electrolytes not only facilitate the electrochemical reactions but also help stabilize the battery's internal environment and extend its lifespan. The Murata Corporation employs advanced material mixing techniques in manufacturing these batteries, optimizing the proportions of anode and cathode materials, and using high-performance separators and antioxidants to enhance overall battery performance, including energy density and stable discharge characteristics.
Despite their many advantages, such as high energy density and low self-discharge rates, which make them the preferred choice for low-power applications like watches and hearing aids, silver oxide batteries have significant limitations. They are single-use, and non-rechargeable, which restricts their range of applications. Additionally, the environmental impact of disposing and recycling used batteries presents ongoing challenges. Nevertheless, the unique properties of silver oxide batteries make them an irreplaceable option in certain applications.
|
Battery
Datasheet
|
Chemistry
Nominal and Cutoff Voltages
|
Capacity
Discharge Current
|
Operating Temperature
Annual Self-Discharge Rate
|
|
Duracell D377/376
|
Silver Oxide
1.55V/1.2V
|
24 mAh, 47kΩ down to 1.2V @20°C
44.8μA @1.54V @20°C
|
0°C to +60°C
<10% @20°C
|
|
Energizer 377/376
|
Silver Oxide
1.55V/1.2V
|
24 mAh, 47kΩ down to 1.2V @21°C
31μA @1.46V 47kΩ @21°C
|
-
~2% @20°C
|
|
Maxell SR626SW
|
Silver Oxide
1.55V/1.2V
|
28 mAh
30μA
|
-10°C to +60°C
-
|
|
muRata SR626
|
Silver Oxide
1.55V/1.2V
|
28 mAh, 30kΩ down to 1.2V @23°C
50μA @1.55V 30kΩ @23°C
|
-10°C to +60°C
-
|
|
Renata 376 High Drain
|
Silver Oxide
1.55V/1.2V
|
27 mAh, 34k8Ω down to 1.2V @20°C
44.5μA @1.55V 34k8Ω @20°C
|
-10°C to +60°C
<10% @20°C
|
|
Renata 377 Low Drain
|
Silver Oxide
1.55V/1.2V
|
24 mAh, 34k8Ω down to 0.9V @20°C
43.7μA @1.55V 34k8Ω @20°C
|
-10°C to +60°C
<5% @20°C
|
|
Varta V 377 MF
|
Silver Oxide
1.55V/1.2V
|
21 mAh, 47kΩ down to 1.2V @20°C
-
|
0°C to +60°C
<10% @20°C
|
Chart
1: Silver Oxide Batteries Comparison Chart - SR626SW, 377, 376 as Example
Definition
Alkaline batteries, a highly efficient type of disposable primary battery, generate power through a reaction between zinc and manganese dioxide. Unlike traditional zinc-carbon batteries that use acidic electrolytes like ammonium chloride or zinc chloride, alkaline batteries employ potassium hydroxide, an alkaline electrolyte. This switch to a more efficient electrolyte allows alkaline batteries to offer both higher energy density and longer shelf life compared to Leclanché cells or zinc chloride types of zinc-carbon batteries.
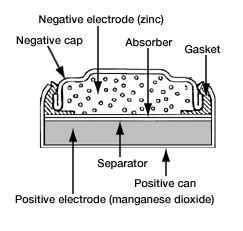
Figure 4: Alkaline Battery Internal Diagram
Work Principle
In the operation of alkaline batteries, the cell itself is central. Here, chemical reactions transform chemical energy into electrical energy that powers external circuits. Specifically, zinc serves as the anode where it readily loses electrons and oxidizes, while manganese dioxide acts as the cathode and is reduced by gaining electrons. The reactions are detailed as follows: at the anode, zinc reacts with water, releasing electrons and forming zinc hydroxide (Zn + 2OH- → Zn(OH)2 + 2e-, with a potential of about -1.28V). At the cathode, manganese dioxide uses these electrons to transform into manganese(III) oxide (2MnO2 + H2O + 2e- → Mn2O3 + 2OH-, with a potential of about +0.15V). The overall battery reaction, Zn + 2MnO2 → Mn2O3 + Zn(OH)2, results in a total potential of approximately 1.43 volts.
Though rare, alkaline batteries can sometimes leak or even burst due to internal short circuits. If a leak occurs, the electrolyte escapes through the broken seal and should be washed off immediately with water to avoid skin irritation. Despite these risks, alkaline batteries are designed to minimize the impact of leaks, typically containing any potential damage to a very limited area and preventing serious harm to users.
Figure 5: Alkaline Battery Reaction Chemical Formula
Types of Batteries
Alkaline batteries come in various forms, distinguished by the type of active materials used in their electrodes, such as nickel-iron (or Edison), nickel-cadmium (or NiFe), silver-zinc, and standard alkaline batteries. They are also categorized based on their assembly as either sealed or unsealed, and by their electrode design, which can be either enclosed in a pocket or open.
Battery Application Scenarios
Alkaline batteries are widely used in numerous devices including toys, flashlights, portable electronic devices, breadboard circuits, and digital cameras. Their high energy density, low internal resistance, and excellent performance in both extreme and mild temperatures allow these batteries to function effectively in both continuous and intermittent applications. Whether operating under high or low discharge conditions, they provide consistent power output. Additionally, the batteries are designed for long shelf life and low leakage rates, ensuring stable size and minimal maintenance needs.
|
Type
|
Typical label
|
Capacity (MAH)
|
Internal resistance (ohms)
|
Weight (grams)
|
Voltage
|
|
Silver-oxide
|
SR621SW SR626SW
|
150–200
|
5 to 15
|
2.3
|
1.55V
|
|
Alkaline
|
LR44, LR1154
LR626
|
100–130
|
3 to 9
|
2.4
|
1.5V
|
Chart
2: Battery Chemistry Comparison Chart
Figure 6: Comparison of SR626SW and SR621SW
When considering choosing silver oxide batteries for watches and sensitive electronic devices, we need to understand the differences in advance because the specific properties and compatibility of different models such as SR626SW and SR621SW are different. Both types are designed to be non-rechargeable, and optimized for devices that require a stable, long-lasting power source to maintain delicate circuit functions.
The main distinctions between the SR626SW and SR621SW focus on their dimensions and discharge properties.
The SR626SW battery is characterized by its size—6.8 mm in diameter and 2.6 mm in height. It also maintains a voltage of 1.55V and offers a battery capacity typically between 25-27 mAh. This particular model is favored in devices that can house its slightly larger size, benefiting from its greater capacity which can extend the operational life of the device.
On the other hand, the SR621SW shares the same diameter of 6.8 mm but stands shorter at 2.1 mm, and it provides a lower capacity range of 18-23 mAh. Although the voltage remains the same at 1.55V, the reduced height and capacity make the SR621SW suitable for smaller devices or those designed specifically for this battery's exact dimensions.
The difference in height of just 0.5 mm between these two batteries might appear negligible but has significant implications for battery fitting and functionality. Devices designed to accommodate an SR626SW might physically fit the smaller SR621SW, but the looser fit could lead to inconsistent electrical contacts, resulting in intermittent power supply or potential device malfunction. Conversely, trying to insert an SR626SW into a compartment designed for an SR621SW could lead to physical strain on both the battery and the device, potentially causing permanent damage or battery leakage.
For optimal device performance and safety, it is critical to select a battery that matches the specified dimensions required by the device manufacturer. Using an SR626SW battery in a device that requires its specific size of 6.8 mm by 2.6 mm ensures that the battery compartment holds the battery securely, maintaining reliable electrical contacts and avoiding issues such as power disruptions or mechanical damage. Always opt for batteries from reputable manufacturers to guarantee the quality and specifications needed for your electronic devices, ensuring they function effectively and safely over their intended lifespan.
|
|
SR621SW
|
SR626SW
|
|
Weight
|
0.32g
|
0.39g
|
|
Capacity
|
23mAh
|
28mAh
|
|
Size / Dimension
|
0.27Dia x 0.08 H 6.8mmx2.0mm
|
0.27Dia x 0.10 H 6.8mmx2.6mm
|
Chart
3: Comparison of Basic Specifications between SR621SW and SR626SW
Figure 7: Alkaline Batteries
After comparing different models of silver oxide batteries, we found that they only differ in size and discharge characteristics. So, what is the difference between silver oxide batteries and alkaline batteries? Today we take SR626SW and LR626 as examples to see what happens.
When comparing silver oxide batteries to alkaline batteries using the examples of SR626SW and LR626, we delve into more than just physical dimensions and discharge characteristics, we explore the suitability of each battery type for specific electronic devices. Both the SR626SW and LR626 share the same physical dimensions, measuring 6.8 mm in height and 2.6 mm in diameter (approximately 0.1023 x 0.2677 inches), which makes them interchangeable in size.
Under industry standards, these batteries are designated differently based on their chemical composition: the LR626 is identified as an alkaline battery, while the SR626 is known as a silver oxide battery. According to the International Electrotechnical Commission (IEC), these batteries are labeled as LR626 for alkaline and SR626 for silver oxide. The American National Standards Institute (ANSI) refers to them as 1176SO batteries. Sometimes, they are also known by shorter two-digit codes: LR66 for alkaline and SR66 for silver oxide.
Manufacturers often use their labeling systems but generally include these standard IEC and ANSI codes along with a brief description of the chemical composition, nominal voltage, and battery equivalents on the packaging. This helps users identify the right type of battery for their needs based on reliable and standardized information.
One crucial difference between these two battery types is how they handle voltage decline. Alkaline batteries, such as the LR626, tend to experience a rapid voltage drop. This makes them less ideal for devices like watches that require a consistent voltage to function properly. Silver oxide batteries, like the SR626, maintain a more stable voltage output over time, which is important for the accurate functioning of timepieces and other sensitive electronic devices.
Due to their small size, the cost per battery is relatively low, making them an economical choice for many users. However, when choosing a battery for devices such as watches, where consistent power output is the key, it is advisable to opt for the SR626 or SR626SW silver oxide batteries. These are specifically designed to provide steady voltage and longer life, ensuring that your device operates reliably without unexpected power interruptions.
|
Chemistry
|
Alkaline
|
Silver-Oxide
|
|
Nominal Voltage
|
1.5V
|
1.55V
|
|
End-Point Voltage
|
1.0V
|
1.2V
|
|
Notes
|
Voltage drops over time
|
Very constant voltage
|
|
Typical Labels
|
LR66, LR626, AG4
|
177, 376, 377, AG4, SG4, SR66, SR626,
SR626SW
|
|
Typical Capacity
|
15-17 mAh
|
25-27 mAh
|
Chart
4: LR626 and SR626 Batteries Comparison Chart
Due to the chemistry and potential environmental impact of small batteries such as LR626 (alkaline) and SR626SW (silver oxide), it is important to properly dispose of used batteries. Here is an enhanced and detailed guide on how to handle the disposal of these batteries responsibly, ensuring safety and sustainability.
Alkaline Batteries (LR626) Disposal Process
Local Regulations Check: Initially, it's crucial to understand your local environmental laws regarding alkaline batteries. Depending on your location, these batteries might be treated as non-hazardous waste and permissible for disposal in regular trash. However, regulations can differ significantly from one region to another, so confirming these details helps ensure compliance with local guidelines.
Recycling Center Identification: Alkaline batteries are not universally accepted in all recycling programs, but they are often included in special waste collection initiatives designed for hazardous or specific types of waste. Identifying a recycling center that accepts these types of batteries can prevent them from ending up in landfills, thereby reducing environmental harm.
Engagement in Battery Recycling Programs: Many retail stores and public facilities offer dedicated battery recycling programs. These programs are tailored to ensure that batteries are disposed of in an environmentally friendly manner, facilitating the recycling of materials that might otherwise be hazardous.
Silver Oxide Batteries (SR626SW) Disposal Process
Handling as Hazardous Waste: Silver oxide batteries, including the SR626SW, contain materials classified as hazardous waste and should never be disposed of with regular household waste due to the risk of environmental contamination.
Utilizing Special Collection Sites: It’s advisable to use municipal or local hazardous waste collection services that cater specifically to the disposal of items like batteries. These facilities ensure that harmful components are properly managed and treated.
Retail Drop-off Points: Many watch shops, electronics stores, and pharmacies provide facilities for dropping off spent silver oxide batteries. These places usually partner with professional recycling services that specialize in the safe handling of hazardous materials, ensuring that the batteries are recycled or disposed of correctly.
General Disposal Tips for Both Battery Types
Securing Battery Terminals: Applying insulating tape over the battery terminals can prevent accidental short circuits, especially when batteries are stored or transported for recycling with other batteries.
Safe Storage Before Disposal: When accumulating batteries for disposal, store them in a location that is cool, dry, and away from any heat sources. It’s important to keep them in a secure place where they cannot be accessed by children or pets, minimizing the risk of accidental ingestion or mishandling.
Avoiding Dangerous Treatment: Batteries should never be burned or punctured. These actions can release toxic chemicals and gases, posing serious health risks and environmental hazards.
Utilizing Mail-back Programs: Some battery manufacturers and community recycling programs provide mail-back services, where consumers can send spent batteries to a facility equipped to handle them appropriately. This option offers convenience and ensures that the batteries are dealt with in a compliant manner.
Adhering to these detailed procedures for disposing of LR626 and SR626SW batteries not only aligns with environmental regulations but also promotes the responsible recycling of potentially hazardous materials. By following local disposal guidelines and opting for recycling whenever possible, you contribute to the reduction of harmful waste in landfills and aid in the preservation of our environment.
Whether opting for the robust and stable power supply of silver oxide batteries or the cost-effective and versatile performance of alkaline batteries, users must consider both the immediate and long-term implications of their choice on device functionality and overall performance. Proper disposal of these batteries is equally crucial, as it involves adhering to environmental regulations and ensuring that the potentially hazardous materials do not adversely affect the ecosystem. By following recommended disposal guidelines and participating in recycling programs, users can mitigate the environmental impact and contribute to sustainability efforts. This responsible approach not only aligns with global environmental goals but also promotes the health and safety of the community, ensuring that future generations continue to benefit from advances in battery technology without compromising the health of our planet.
Frequently Asked Questions [FAQ]
1. What battery is equivalent to SR626SW?
The SR626SW battery equivalents include 377, 376, AG4, and SG4.
2. What is an SR626SW battery?
The SR626SW is a small, button-type silver oxide battery commonly used in watches and small electronic devices due to its stable voltage and long shelf life.
3. Is silver oxide battery the same as alkaline?
No, silver oxide batteries and alkaline batteries are not the same. Silver oxide batteries use silver oxide as the cathode and provide more consistent voltage and higher energy density compared to alkaline batteries, which use manganese dioxide as the cathode.
4. What is the advantage of a silver oxide battery?
Silver oxide batteries offer a higher energy density and more stable voltage output over their lifetime, making them ideal for precision devices like watches and medical instruments.
5. Can you interchange alkaline and silver oxide batteries?
Yes, in many cases, alkaline and silver oxide batteries can be interchanged if they share the same size and voltage specifications, but performance differences such as voltage consistency and lifespan should be considered.
Share: