Discovering the Pros and Cons of Lithium-Ion Batteries
In an era marked by rapid technological advancements and increasing environmental consciousness, lithium-ion batteries have emerged as a cornerstone in developing energy storage solutions. These batteries are integral to a myriad of applications, from portable electronics to electric vehicles, and are ultimate in the transition towards more sustainable energy systems. The popularity of lithium-ion batteries stems from their superior energy density, efficiency, and rechargeability compared to traditional battery technologies like nickel-cadmium or lead-acid.This article delves into the sophisticated mechanics of lithium-ion battery operation, exploring their composition, advantages, and challenges. It further discusses the environmental impacts associated with their use and disposal, contrasting their features with those of lead-acid batteries to underscore their relevance in contemporary and future energy scenarios.
Catalog
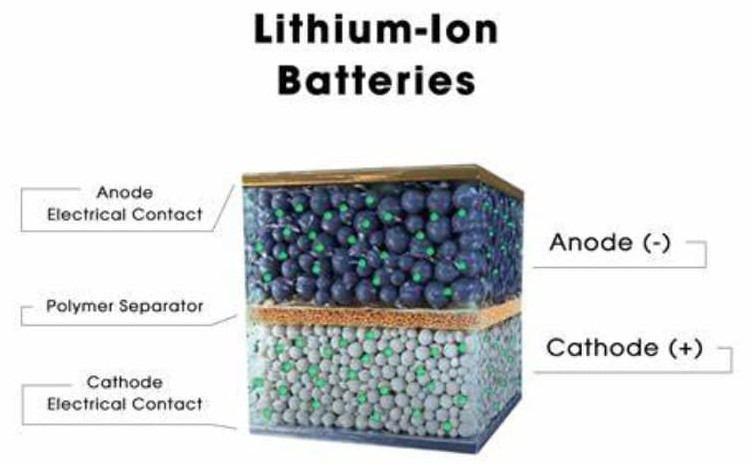
Figure 1: Lithium-ion Batteries
The Basics of Lithium-Ion Batteries
Lithium-ion batteries play a significant role in powering a wide range of modern devices, from smartphones to electric vehicles. These batteries are preferred because they are compact, lightweight, and capable of recharging quickly, making them more efficient and user-friendly compared to traditional nickel-based and lead-acid batteries.
A lithium-ion battery is composed of four needed components: the anode, cathode, separator, and electrolyte. The anode and cathode are dynamic for the flow of electrons during the battery’s discharge process. The separator serves as a safety barrier, ensuring that the anode and cathode do not come into direct contact, which helps prevent short circuits while maintaining ionic balance. The electrolyte facilitates the movement of lithium ions between the anode and cathode during both the charging and discharging phases.
This interplay among the anode, cathode, separator, and electrolyte allows lithium-ion batteries to store energy effectively within their tightly packed layers. As a result, these batteries deliver reliable performance across a variety of demanding applications.
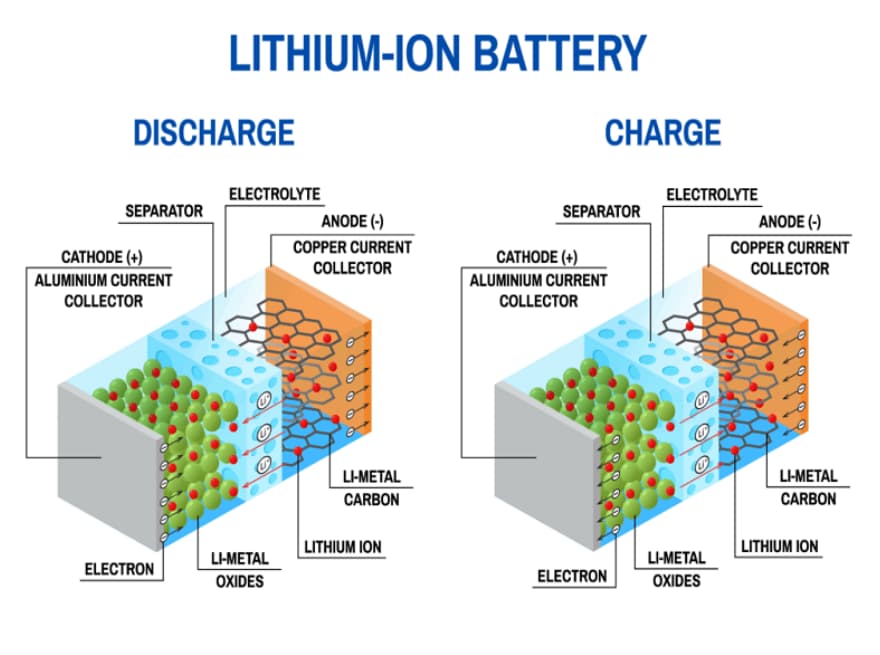
Figure 2: Mechanism of Lithium-Ion Battery Operation
Mechanism of Lithium-Ion Battery Operation
Lithium-ion batteries work by moving lithium ions between the anode and cathode through an electrolyte. The anode is typically made of carbon-based materials like graphite, chosen for their conductivity and stability. The cathode, on the other hand, is usually made from metal oxides such as lithium cobalt oxide or lithium iron phosphate, each offering different advantages in terms of energy density and safety.
When the battery discharges, lithium ions move from the anode to the cathode via the electrolyte. This movement of ions causes free electrons to be released at the anode. These electrons then flow through an external circuit, generating the electric current that powers devices like smartphones or electric cars. The separator, a porous membrane within the battery, is required during this process. It prevents the electrons from directly traveling from the anode to the cathode, which avoids short circuits and ensures safe operation.
During recharging, the process reverses: lithium ions are pushed back to the anode, restoring the battery’s capacity for the next use. This back-and-forth movement of ions is what makes lithium-ion batteries efficient, providing consistent and reliable power to a wide range of electronic devices.
Advantages of Lithium-ion Batteries
Lithium-ion batteries play a valuable role in powering modern technology, from smartphones to electric vehicles, thanks to their numerous advantages.
|
Advantages of Lithium-ion Batteries |
|
|
High Energy Density |
Lithium-ion batteries can store a large
amount of energy in a small space. This high energy density is especially
beneficial for portable electronics like smartphones and laptops, allowing
these devices to run longer between charges while remaining lightweight and
compact. |
|
Low Self-Discharge Rate |
One of the standout features of
lithium-ion batteries is their low self-discharge rate. Unlike older battery
technologies like nickel-cadmium (Ni-Cad) or nickel-metal hydride (NiMH),
which lose a significant amount of charge when not in use, lithium-ion batteries
retain their charge for much longer. This makes them ideal for devices that
need to stay charged over long periods, such as emergency equipment or
seasonal gadgets. |
|
No Memory Effect |
Lithium-ion batteries are free from the
memory effect, a problem seen in some other types of batteries, like Ni-Cad.
With these older batteries, repeated partial discharges could reduce their
overall capacity unless they were fully discharged before recharging.
Lithium-ion batteries don’t have this issue, allowing them to be recharged at
any point without affecting their capacity, which simplifies their
maintenance and prolongs their lifespan. |
|
Higher Cell Voltage |
Lithium-ion batteries typically offer a
higher cell voltage, around 3.6 volts per cell, compared to 1.2 volts for
NiMH or Ni-Cad. This higher voltage means that fewer cells are needed in a
battery pack to achieve the desired overall voltage, which simplifies the
design and can reduce the weight and cost of the battery packs. |
|
Versatility and Scalability |
Lithium-ion technology is versatile and
scalable, making it suitable for a wide range of applications, from small
medical devices to large-scale energy storage systems. Manufacturers can
tailor the chemistry and configuration of lithium-ion batteries to optimize
performance for specific needs, enhancing the power output of electric
vehicles or the energy efficiency of portable electronics. |
|
Reduced Environmental Impact |
Compared to batteries containing heavy
metals like lead or nickel, lithium-ion batteries use less harmful materials,
which can often be recycled. When disposed of properly, they have a lower
environmental impact, making them a more sustainable choice. |
Disadvantages of Lithium-ion Battery
While lithium-ion batteries are basic in modern energy storage and power systems, they come with several notable disadvantages that can limit their effectiveness and broader use.
|
Lithium-ion Battery Disadvantages |
|
|
Complex Protection Requirements |
Lithium-ion batteries need advanced
protection circuits to operate safely. These circuits are used for preventing
overcharging and deep discharge, which can lead to a dangerous condition
called thermal runaway, where the battery can overheat uncontrollably, posing
risks of fires or explosions. The need for these Battery Management Systems
(BMS) complicates the design process and increases production costs, making
the batteries more expensive to manufacture and integrate into products. |
|
Degradation and Lifespan Issues |
Over time, lithium-ion batteries
experience a decline in capacity and efficiency, especially with repeated
charging cycles. This degradation means they need to be replaced more
frequently than some other battery types, leading to higher long-term costs and
more waste. In addition, disposing of these batteries poses environmental
challenges due to the hazardous materials they contain. |
|
Transportation and Regulatory
Challenges |
Lithium-ion batteries are prone to short
circuits and fires, making their transportation, particularly by air, risky.
This has led to strict regulations requiring special packaging and handling,
which complicates logistics and increases shipping costs. These added
expenses impact the efficiency of distribution and raise operational costs
for businesses that rely on lithium-ion technology. |
|
High Production Costs |
The production of lithium-ion batteries
involves advanced materials and technology, contributing to their high cost.
These expenses are often passed on to consumers, making products that use
these batteries more expensive. Although research is ongoing to reduce
production costs and improve performance, the high initial investment remains
a barrier to wider adoption, particularly in price-sensitive markets. |
|
Environmental and Ethical Concerns |
The extraction of lithium and other
metals used in these batteries can cause significant environmental harm, such
as water pollution and disruption of ecosystems. In addition, ethical issues
surrounding mining practices, including labor rights and community
displacement, add further complexity to the sustainability of lithium-ion
batteries. |
Lithium-ion Battery Variants
Lithium-ion batteries are useful in today's technology-driven world, and they come in several variants, each designed for specific applications based on their chemical makeup.

Figure 3: Lithium Iron Phosphate (LiFePO4)
LiFePO4 batteries are known for their outstanding safety and long lifespan. Their chemical stability significantly reduces the risk of overheating, making them a safer choice compared to other types. This makes them ideal for applications that require high reliability, such as electric vehicles (EVs) and stationary energy storage systems.

Figure 4: Lithium Cobalt Oxide (LiCoO2)
LiCoO2 batteries are commonly used in personal electronics like smartphones and laptops due to their high energy density. This feature allows these devices to have longer runtimes while keeping a slim, lightweight design. However, these batteries are more expensive and less thermally stable, which limits their use to smaller devices rather than large-scale energy systems.

Figure 5: Lithium Manganese Oxide (LiMn2O4)
LiMn2O4 batteries strike a good balance between energy density, power output, and safety. The addition of manganese improves thermal stability and makes these batteries a more cost-effective solution compared to LiCoO2. As a result, they are often used in consumer electronics and electric power tools.

Figure 6: Lithium Nickel Manganese Cobalt Oxide (LiNiMnCoO2 or NMC)
NMC batteries are among the most versatile lithium-ion variants, offering a high energy density combined with better stability. These features make them suitable for a wide range of applications, from electric vehicles to portable electronics. Ongoing advancements in NMC technology are continuously improving their energy capacity, safety, and lifespan, meeting the increasing demands of both automotive and renewable energy storage sectors.

Figure 7: Lithium Nickel Cobalt Aluminum Oxide (LiNiCoAlO2 or NCA)
NCA batteries are similar to NMC in providing high energy densities and are used in high-performance applications, such as advanced electric vehicles and aerospace technologies. The inclusion of aluminum in their composition enhances their overall stability and extends their lifespan.

Figure 8: Lithium Titanate (Li2TiO3)
Lithium titanate batteries are known for their fast-charging capabilities and long cycle life. These batteries are particularly well-suited for situations where quick recharging is tough, such as in public transportation and backup power systems. Although they have lower energy densities, their durability and safety make them an excellent choice for specific high-demand applications.
Diverse Uses of Lithium-Ion Batteries
Lithium-ion batteries are dominant in driving technological advancement and promoting sustainability across various sectors. Their high energy density, rapid charging capabilities, and long lifespan make them requisite in many applications.
Emergency Power Systems: Lithium-ion batteries are increasingly being used in Uninterruptible Power Supplies (UPS) for serious systems in hospitals, data centers, and other facilities where constant power is a must. These batteries offer fast response times and quick recharging, which significantly reduces the risk of power outages compared to traditional lead-acid batteries. In addition, they provide a more stable power output, which is dynamic for maintaining sensitive electronic equipment.
Renewable Energy Storage: In renewable energy systems, lithium-ion batteries are dynamic for storing excess energy generated by solar panels and wind turbines. This stored energy can be used during periods of low production, such as nighttime or calm weather, ensuring a consistent energy supply. This capability is suitable for stabilizing power grids and supporting the transition to renewable energy sources, reducing reliance on fossil fuels.
Electric Transportation: Lithium-ion batteries are at the heart of electric transportation, powering everything from electric cars and buses to bicycles and scooters. These batteries have enabled the development of electric vehicles (EVs) with longer ranges and shorter charging times, making EVs a more practical and appealing choice for consumers. The widespread adoption of lithium-ion batteries in transportation is noteworthy for reducing greenhouse gas emissions and decreasing dependence on oil.
Consumer Electronics: Lithium-ion batteries are fundamental to modern consumer electronics, powering smartphones, laptops, tablets, and wearable devices. Their ability to store a large amount of energy in a small, lightweight package makes them perfect for the demands of today’s mobile, digital lifestyle. This efficiency not only enhances device performance and user experience but also drives the development of increasingly advanced technologies.
Industrial Applications: Lithium-ion batteries are also making a significant impact in industrial settings, powering tools, machinery, and automation systems that require reliable and long-lasting energy sources. Their durability and capacity to deliver high currents on demand make them ideal for heavy-duty applications in challenging environments.
Aerospace and Marine Sectors: In aerospace, lithium-ion batteries power satellites, drones, and other aviation technologies offering a superior power-to-weight ratio compared to traditional batteries. Similarly, in the marine industry, these batteries are used in electric and hybrid vessels, improving efficiency and reducing emissions in everything from small boats to large ships.
The Eco-Costs of Lithium-Ion Batteries
While lithium-ion batteries are insistent on advancing clean technology, they also raise significant environmental concerns. The extraction of lithium, a dynamic component, requires large amounts of water and often leads to severe ecological damage, especially in arid regions where water is already scarce. This extraction process damages local ecosystems and depletes water resources for communities and wildlife.
In addition, the disposal of lithium-ion batteries at the end of their life cycle poses serious environmental risks. If not properly managed, these batteries can release toxic metals like cobalt and nickel into the soil and water, leading to contamination that threatens ecosystems and human health.
To mitigate these environmental impacts, a holistic approach to the lifecycle of lithium-ion batteries is required. This includes regulating mining practices to reduce ecological harm, promoting advanced recycling technologies to recover valuable materials, and developing alternative battery technologies with smaller environmental footprints. These steps are dangerous for minimizing the ecological impact of lithium-ion batteries while maintaining their role in modern technology.
Figure 9: Lithium-ion and Lead-Acid Batteries
Comparison of Lithium-Ion and Lead-Acid Batteries
Lithium-ion and lead-acid batteries are widely used across various industries, each with distinct characteristics suited to different applications.
• Weight and Efficiency
Lithium-ion batteries are much lighter than lead-acid batteries, making them ideal for applications where efficiency and mobility are settling, such as in electric vehicles and portable electronics. The reduced weight of lithium-ion batteries leads to lower energy consumption, resulting in extended driving ranges and better performance in vehicles.
• Battery Protection and Management
Lithium-ion batteries come with advanced battery management systems (BMS) that carefully regulate their operation. These systems monitor key factors like temperature, voltage, and current, ensuring optimal performance and preventing dangerous situations like overcharging or deep discharges. In contrast, lead-acid batteries have simpler protection systems and are more prone to damage from such issues, which can shorten their lifespan.
• Charging Characteristics
Lithium-ion batteries charge much faster than lead-acid batteries and can handle partial charge cycles without needing a full discharge before recharging. This quick charging capability is particularly useful in consumer electronics and electric vehicles. In addition, lithium-ion batteries retain their charge longer when not in use, with minimal self-discharge, making them more reliable for seasonal or intermittent use.
• Energy Density and Power Delivery
Lithium-ion batteries offer a higher energy density, delivering more energy per unit of weight compared to lead-acid batteries. This allows for smaller, lighter batteries that still provide the same power output as larger, heavier lead-acid batteries. The higher energy density also translates to better performance in high-drain applications like electric vehicles and large-scale energy storage systems. While lead-acid batteries can deliver substantial power, they do so at the cost of greater weight and volume.
• Lifespan and Sustainability
Lithium-ion batteries generally last longer than lead-acid batteries, with the ability to endure more charge-discharge cycles before their performance deteriorates. While the environmental impact of lithium-ion batteries is significant, it can be mitigated through advancing recycling technologies. Lead-acid batteries, though highly recyclable, tend to have a shorter lifespan and a larger environmental footprint due to the need for more frequent replacements.
• Cost Considerations
Initially, lithium-ion batteries are more expensive to produce than lead-acid batteries, owing to their complex chemistry and manufacturing processes. However, their longer lifespan and lower maintenance requirements can result in a lower total cost of ownership over time, especially in applications where their benefits are fully leveraged.
Conclusion
Lithium-ion batteries represent a significant leap forward in battery technology, offering enhancements that are hard for modern technology and environmental sustainability. Their high energy density, efficiency, and versatility make them suitable for a wide array of applications, ranging from everyday consumer electronics to large-scale renewable energy storage systems. However, the advantages of lithium-ion batteries are tempered by challenges such as complex production demands, safety concerns, and environmental implications stemming from their materials and disposal.
Addressing these challenges requires ongoing technological innovation and regulatory oversight to optimize their performance and mitigate their ecological impact. As the technology evolves, the potential for lithium-ion batteries to power a cleaner, more efficient future remains vast, underscoring the need for continued research and adaptation in this dynamic field.
Frequently Asked Questions [FAQ]
1. What are the benefits of lithium-ion batteries?
High Energy Density: They can store a lot of energy in a small space, making them ideal for portable devices like smartphones and laptops.
Lightweight: Lithium-ion batteries are lighter than other types, such as lead-acid batteries, which are used for applications like electric vehicles and portable electronics.
No Memory Effect: They don't require a full discharge before recharging, which means they can be topped up at any time without reducing their effective capacity over time.
Long Lifespan: They can handle hundreds to thousands of charge and discharge cycles before their capacity falls significantly.
Fast Charging: Lithium-ion batteries charge faster than many other types of rechargeable batteries.
2. What is the biggest problem with lithium batteries?
Safety Risks: They can pose fire and explosion risks if damaged, overheated, or improperly charged due to their flammable electrolyte and high energy density.
3. What are the negative effects of lithium-ion batteries?
Environmental Impact: The mining of lithium, needed for these batteries, has significant environmental impacts, including water pollution and habitat destruction.
Resource Scarcity: Lithium and other grave materials like cobalt are limited and primarily sourced from a few regions, raising concerns about sustainability and geopolitical tensions.
Disposal Issues: Improper disposal can lead to harmful chemicals leaching into the environment. Recycling processes are in place but not yet widespread or fully efficient.
4. How long will a lithium battery last?
Typically, lithium-ion batteries last for 2 to 3 years or about 300 to 500 charge cycles, whichever comes first. In terms of daily use, this often translates to around 1,000 full charge-discharge cycles before the battery capacity degrades to 80% of its original capacity.
5. How to make a lithium-ion battery last longer?
Avoid Full Discharges: Frequently discharging the battery to 0% can shorten its lifespan. Try to keep the charge between 20% and 80%.
Keep It Cool: High temperatures can degrade the battery faster. Store and use the battery in a cool, shaded place when possible.
Use Appropriate Chargers: Using a charger that matches the specifications recommended by the manufacturer can help maintain battery health.
Reduce Charge Speed: Fast charging can be convenient but may increase wear and tear. When time allows, opt for slower charging methods.
Minimize Exposure to Extreme Conditions: Both high heat and very cold temperatures can harm battery life. Keep devices with lithium-ion batteries away from extreme temperatures.
About us
ALLELCO LIMITED
Read more
Quick inquiry
Please send an inquiry, we will respond immediately.
Inclusive Guide to FM Slope Detection and Demodulation Technology
on August 22th
Inductor, Choke Coil & Transformer Circuit Symbols
on August 22th
Popular Posts
-
What is GND in the circuit?
on January 1th 2898
-

RJ-45 Connector Guide: RJ-45 Connector Color Codes, Wiring Schemes, R-J45 Applications, RJ-45 Datasheets
on January 1th 2463
-

Fiber Connector Types: SC Vs LC And LC Vs MTP
on January 1th 2060
-
Understanding Power Supply Voltages in Electronics VCC, VDD, VEE, VSS, and GND
on November 7th 1832
-

Comparison Between DB9 and RS232
on January 1th 1746
-

What Is An LR44 Battery?
Electricity, that ubiquitous force, quietly permeates every aspect of our daily lives, from trivial gadgets to life-threatening medical equipment, it plays a silent role. However, truly grasping this energy, especially how to store and efficiently output it, is no easy task. It is against this background that this article will focus on a type of coin cell battery that may seem insignificant on the...on January 1th 1699
-
Understanding the Fundamentals:Inductance Resistance, andCapacitance
In the intricate dance of electrical engineering, a trio of fundamental elements takes center stage: inductance, resistance, and capacitance. Each bears unique traits that dictate the dynamic rhythms of electronic circuits. Here, we embark on a journey to decipher the complexities of these components, to uncover their distinct roles and practical uses within the vast electrical orchestra. Inductan...on January 1th 1641
-

CR2430 Battery Comprehensive Guide: Specifications, Applications and Comparison to CR2032 Batteries
What is CR2430 battery ?Benefits of CR2430 BatteriesNormCR2430 Battery ApplicationsCR2430 EquivalentCR2430 VS CR2032Battery CR2430 SizeWhat to look for when buying the CR2430 and equivalentsData Sheet PDFFrequently Asked Questions Batteries are the heart of small electronic devices. Among the many types available, coin cells play a crucial role, commonly found in calculators, remote controls, and ...on January 1th 1517
-
What Is RF and Why Do We Use It?
Radio Frequency (RF) technology is a key part of modern wireless communication, enabling data transmission over long distances without physical connections. This article delves into the basics of RF, explaining how electromagnetic radiation (EMR) makes RF communication possible. We will explore the principles of EMR, the creation and control of RF signals, and their wide-ranging uses. The article ...on January 1th 1510
-

CR2450 vs CR2032: Can The Battery Be Used Instead?
Lithium manganese batteries do have some similarities with other lithium batteries. High energy density and long service life are the characteristics they have in common. This kind of battery has won the trust and favor of many consumers because of its unique safety. Expensive tech gadgets? Small appliances in our homes? Look around and you'll see them everywhere. Among these many lithium-manganes...on January 1th 1487



















































