Static Electricity
Static electricity, a phenomenon known since ancient times for its fascinating effects of attraction and repulsion after objects are rubbed together. Early experiments with materials like glass, silk, paraffin wax, and wool helped build the understanding of electrostatics. Significant contributions from historical figures such as Charles Dufay and Benjamin Franklin helped develop theories about the invisible forces at play, eventually identifying electric charge as the movement of electrons. The discovery of the Leyden jar in 1745 and advancements by inventors like Otto von Guericke enabled the generation of larger static charges, further advancing the study of electrostatics. Charles Coulomb's work on the forces between charged particles provided a deeper understanding of these phenomena. This article delves into the history, theories, and practical applications of static electricity, highlighting its impact on scientific thought and technological innovation.
Catalog
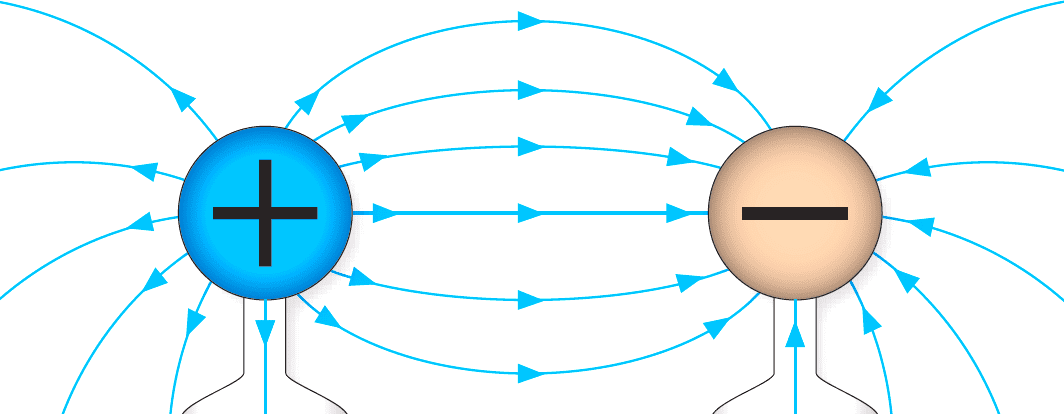
Figure 1: Static Electricity
Historical Discoveries
Centuries ago, it was noticed that certain materials, such as glass and silk, would attract each other after being rubbed together. This interesting event was not limited to glass and silk; other combinations, like paraffin wax and wool, showed similar behavior. Experimenters saw that while rubbed materials of different types attracted each other, the same materials pushed each other away.
Further investigations showed that any material demonstrating attraction or repulsion after being rubbed could be placed into one of two groups: attracted to glass and repelled by wax, or repelled by glass and attracted to wax. This grouping suggested that materials fell into two clear categories based on their electrical properties.
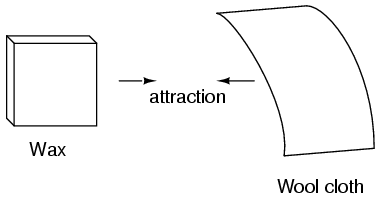
Figure 2: Wax And Wool Cloth Attraction
Early Theories and Experiments
Invisible changes causing attraction or repulsion led early experimenters to think about the transfer of invisible "fluids" during rubbing. Charles Dufay showed that rubbing certain pairs of objects created two distinct types of changes, leading to either attraction or repulsion between the materials. Dufay's findings demonstrated that materials could be grouped based on their behavior after rubbing: some materials attracted each other, while others repelled each other.
Building on these observations, Benjamin Franklin proposed a theory involving a single type of fluid. According to Franklin, rubbing objects together did not involve two different fluids but rather caused an imbalance of a single fluid, which he called an electric charge. Objects could have either too much (+) or too little (-) of this fluid. Franklin's terms for this were "positive charge" (+) for having too much and "negative charge" (-) for having too little.
Franklin's hypothesis provided a simpler way to understand static electricity. He suggested that the attraction and repulsion observed between materials were due to the imbalance of this single electric charge. This idea laid the groundwork for further study and the eventual identification of electric charge as the movement of electrons.
Franklin's Contributions
Benjamin Franklin did experiments with materials like wax and wool to understand static electricity. He thought that rubbing these materials together moved an invisible fluid between them. He believed that wool took some of this fluid from the wax, creating an imbalance that made the two materials attract each other.
Franklin called the charge on the wax "negative" because he thought it had less of this fluid. He called the charge on the wool "positive" because he thought it had more of the fluid. Even though we now know that this "fluid" is actually the movement of electrons, Franklin's terms "positive" and "negative" charges are still used. This terminology stays because it accurately describes the direction of electron flow: from a material with more electrons (-) to one with fewer electrons (+).
Quantifying Electrical Charge
In the 1780s, French physicist Charles Coulomb measured electrical charge using a torsional balance. His experiments led to the definition of the coulomb, a unit of electrical charge. Coulomb's work showed that the force between two point charges was proportional to the product of their charges and inversely proportional to the square of the distance between them. One coulomb equals the charge of approximately 6.25 × 10^18 electrons, and one electron has a charge of about 0.00000000000000000016 coulombs.
The Composition of the Atom
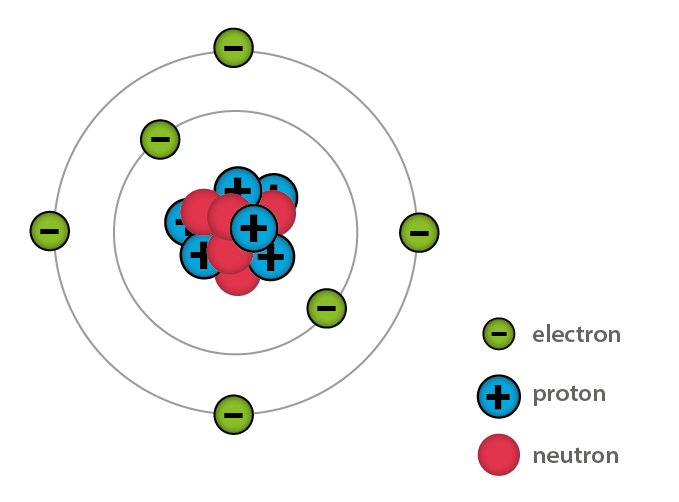
Figure 3: Composition of the Atom
Further experiments showed that all matter is made of atoms, which consist of three main particles: protons, neutrons, and electrons. Protons have a positive (+) charge, electrons have a negative (-) charge, and neutrons have no charge.
The structure of an atom includes the nucleus and electron shells. The nucleus, located at the center of the atom, contains protons and neutrons, which are tightly bound together. This tight binding gives the nucleus its stability and defines the elemental identity of the atom. Changing the number of protons turns the atom into a different element.
Electrons orbit the nucleus in regions called electron shells. Unlike protons and neutrons, electrons are not tightly bound to the nucleus. They can be easily moved by various forces, leading to an electrical imbalance. When electrons move from one atom to another, this creates an electric charge.
The ability of electrons to move more freely compared to protons and neutrons is key to the phenomenon of static electricity. When certain materials are rubbed together, electrons are transferred from one material to another, causing one object to become positively charged (lacking electrons) and the other to become negatively charged (having extra electrons). This movement of electrons is the basis of static electricity.
Static Electricity Explained
Static electricity happens because there is an imbalance of electrons between objects. When certain materials are rubbed together, electrons—negatively charged particles—move from one material to another. This transfer causes one object to gain electrons, becoming negatively charged, and the other to lose electrons, becoming positively charged. This movement of electrons creates an imbalance of electrical charge, with one material having more electrons (negative charge) and the other having fewer electrons (positive charge).
Objects with opposite charges attract each other, while objects with the same charge repel each other. This is why a balloon rubbed on hair sticks to a wall. The balloon, now negatively charged from gaining electrons from the hair, is attracted to the neutral or positively charged wall.
Everyday examples of static electricity include the balloon and hair scenario and clothes in a dryer. In the case of the balloon, rubbing it on hair transfers electrons, making the balloon negatively charged and causing it to stick to a neutral wall. Similarly, in a clothes dryer, friction between clothes transfers electrons, causing static cling as clothes stick together due to opposite charges.
The Triboelectric Effect
Figure 4: Triboelectric Effect
The triboelectric effect happens when two different materials are rubbed together, causing electrons to move from one material to the other. This movement makes one material positively charged (because it loses electrons) and the other negatively charged (because it gains electrons).
This effect explains many everyday experiences of static electricity. For example, when you rub a balloon on your hair, electrons move from your hair to the balloon. As a result, your hair becomes positively charged, and the balloon becomes negatively charged. The opposite charges attract each other, causing your hair to stick to the balloon.
The triboelectric effect depends on the properties of the materials involved. Some materials easily give up electrons, while others attract and hold onto them. This tendency is described by the triboelectric series, which ranks materials based on how likely they are to gain or lose electrons.
When two materials from opposite ends of the triboelectric series are rubbed together, the transfer of electrons is more significant, leading to a stronger static charge. For example, rubbing glass (which tends to lose electrons) with silk (which tends to gain electrons) results in a noticeable static charge.
Practical Applications
Even though it is often seen as annoying, static electricity has many useful uses:
Xerographic Printing
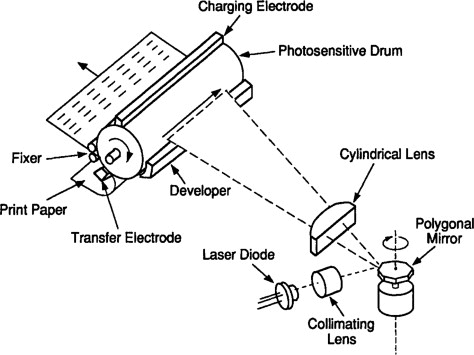
Figure 5: Xerographic Printing
Xerographic printing relies on static electricity to work. This technology is used in photocopiers and laser printers. Here’s a detailed look at how it functions:
A photoconductive drum inside the copier or printer is first given a static charge. This drum can hold an electric charge and reacts to light. When an image of the document to be copied is projected onto the drum, the light makes the static charge go away in the areas exposed to it, while the charge stays in the dark areas where there is no light.
Next, toner, which is a fine powder with a positive charge, is sprinkled onto the drum. The positively charged toner sticks to the negatively charged areas of the drum where the charge has not been neutralized by the light. This creates a powdery image of the document on the drum.
The drum then rolls over a piece of paper, transferring the toner image onto the paper. Finally, the paper passes through a pair of heated rollers called a fuser. The heat and pressure from the fuser melt the toner particles, making them stick to the paper permanently.
This entire process happens very quickly and efficiently, allowing for the rapid production of high-quality copies and prints. The use of static electricity in xerographic printing is a brilliant application of basic scientific principles, turning them into a practical technology that we use every day.
Electrostatic Air Filters
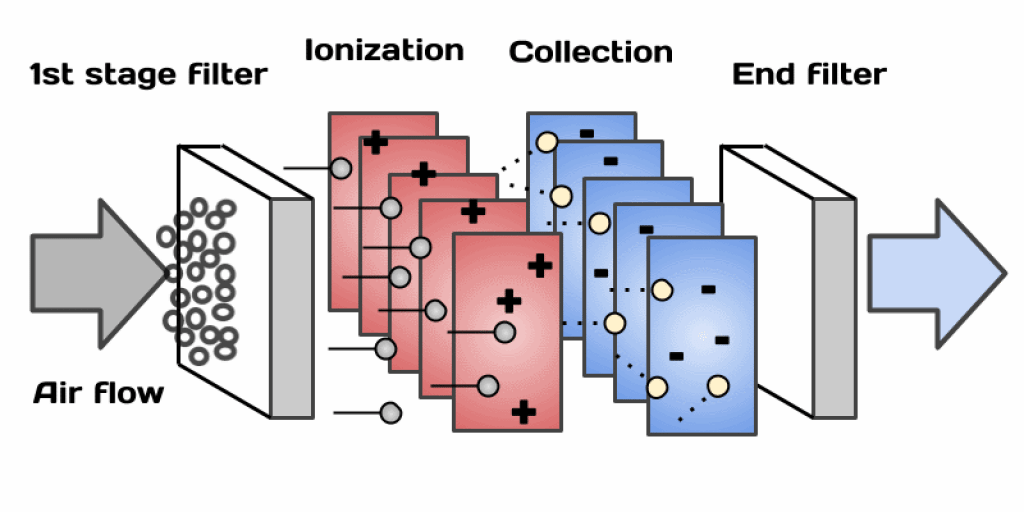
Figure 6: Electrostatic Air Filters
Electrostatic air filters use static electricity to clean the air by removing particles like dust, pollen, and other contaminants. Here's how they work in more detail:
First, the filter becomes charged with static electricity. This can happen in a couple of ways. One common method is to use an electric field to charge the filter material. Another way is to pass air through a grid of wires that charge the particles in the air as they pass through.
Once the filter is charged, it attracts and captures particles from the air. The charged filter works like a magnet for dust and other small particles. When these particles come close to the filter, the electrostatic charge pulls them in, causing them to stick to the filter. This makes the air passing through much cleaner.
Electrostatic air filters are very effective because they can capture very small particles that other types of filters might miss. This includes not just dust and pollen but also smoke, bacteria, and even some viruses. Because of this high efficiency, they are often used in places where air quality matters a lot, such as in homes with allergy sufferers or in industrial settings where clean air is needed for both health and product quality.
One of the main benefits of electrostatic air filters is that they can be reused. Instead of replacing the filter every time it gets dirty, you can clean it and put it back. This makes them more environmentally friendly and cost-effective over time. However, it is necessary to clean the filter regularly to keep it working well. If the filter gets too dirty, it can't hold any more particles, and the air quality will suffer.
Van de Graaff Generator
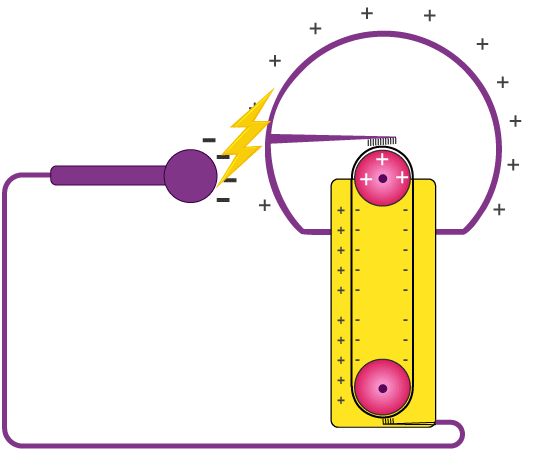
Figure 7: Van de Graaff Generator
The Van de Graaff generator, created by physicist Robert J. Van de Graaff in the 1930s, is a machine that produces high voltages using static electricity. This device works by moving an electric charge to a metal sphere through a belt. As the belt moves, it carries the charge to the sphere, where it builds up. This process can generate voltages reaching millions of volts, making the Van de Graaff generator very useful for scientific experiments, especially in particle physics, where it is used to speed up particles.
Michael Faraday's experiments in 1832 showed that static electricity is the same as the electricity made by batteries and generators. Faraday demonstrated that both types of electricity could cause the same chemical and physical effects, such as breaking down chemical compounds and creating magnetic fields. His work showed that all types of electricity come from the same basic phenomenon: the movement of electric charge.
The Van de Graaff generator and Faraday's discoveries have greatly influenced our understanding of electricity. The Van de Graaff generator, with its ability to produce high voltages, has been very helpful in advancing research in particle physics. It allows scientists to speed up particles to high speeds, making it possible to study the basic parts of matter and forces.
Faraday's work, on the other hand, laid the groundwork for our understanding of electricity as a single phenomenon. By proving that static and current electricity are basically the same, he connected different types of electrical phenomena. This understanding has been very helpful in the development of various electrical technologies and applications.
Together, these developments show how scientific discoveries are connected to their practical uses. The Van de Graaff generator and Faraday's experiments have not only deepened our theoretical knowledge of electricity but also led to significant technological advancements.
Electrostatics on a Large Scale
In the mid-1600s, inventors started making electrostatic machines that could create much larger charges than those made by simple rubbing. These machines worked using rotating wheels or cylinders made of insulating materials like glass or sulfur. Constant friction with materials such as cloth or fur electrified these materials, allowing for the production of significant electrical sparks and static charges.
One of the earliest known electrostatic machines was built in 1660 by Otto von Guericke in Magdeburg, Germany. Guericke's machine used a rotating sulfur ball that, when rubbed, could produce strong static charges. This invention marked a major advancement in the study of electrostatics.
The invention of the Leyden jar in 1745 by Pieter van Musschenbroch in Leyden, Holland, further transformed the field. A Leyden jar is basically a glass jar partially coated inside and out with metal foil, allowing it to store a large static charge. By connecting two Leyden jars to an electrostatic machine—one to hold a negative charge and the other a positive charge—it became possible to accumulate large amounts of static electricity.
These advancements allowed for the generation of much larger and more dangerous sparks. For example, in a high school physics experiment, an electrostatic machine with Leyden jars could produce a spark 15 centimeters long, causing temporary paralysis if accidentally discharged through a human hand.
The pursuit of generating ever-larger electrostatic charges became somewhat of a scientific trend in the mid-18th century. In America, Benjamin Franklin used electrostatic machines to electrocute turkeys for his dinner table. In 1750, French physicist Abbe Nollet conducted a dramatic demonstration by having over a thousand Carthusian monks hold hands in a circle while he discharged a massive Leyden jar. The simultaneous jump of all the monks showed the instantaneous speed of electrical discharge.
The similarity between the sparks produced by electrostatic machines and lightning bolts did not go unnoticed. In June 1752, Benjamin Franklin conducted his famous kite experiment to test whether lightning was indeed a giant electric spark. During a thunderstorm, Franklin and his son used a kite to transfer the electric charge from storm clouds to a Leyden jar, conclusively proving that lightning was an electrical phenomenon. This experiment led to the invention of the lightning rod, a device that protects buildings by safely conducting lightning strikes to the ground.
Franklin's theoretical contributions were also very meaningful. He introduced the terms "positive" and "negative" for electric charges and showed through experiments that the amount of negative charge on a rubbed object is exactly equal to the positive charge on the object doing the rubbing. This was a big step towards the idea of conservation of charge, which says that the total electric charge in an isolated system stays the same.
Lightning and Electrostatics
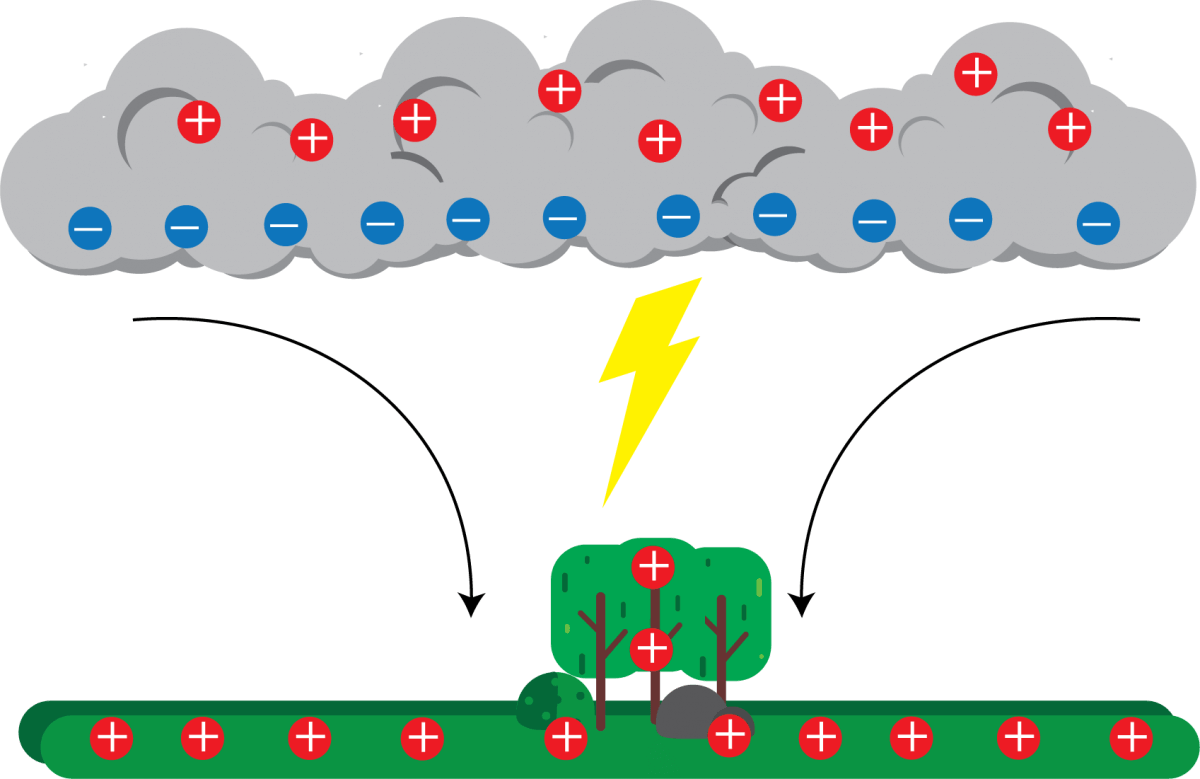
Figure 8: Lightning and Electrostatics
In 1752, Benjamin Franklin did his well-known kite experiment to show that lightning is an electrical discharge. During a thunderstorm, Franklin flew a kite with a metal key attached to the string. When lightning hit the kite, the key became electrified, proving his idea was right. This experiment showed that lightning is a form of electrical discharge, like the sparks made by static electricity.
After this big discovery, Franklin invented the lightning rod. The lightning rod is a simple but effective tool made to protect buildings from lightning strikes. It has a pointed metal rod placed at the highest point of a building, connected to the ground with a conductive wire. When lightning strikes, the rod safely directs the electrical charge down the wire and into the earth, stopping damage to the building.
Franklin's lightning rod works because the sharp point of the rod makes the air around it ionize, creating an easy path for the electrical discharge. This path directs the lightning's energy away from the building, lowering the risk of fire and structural damage. Franklin's invention was a big step forward in our understanding and handling of natural electrical events, providing a useful solution to a potentially very harmful problem.
Coulomb's Law
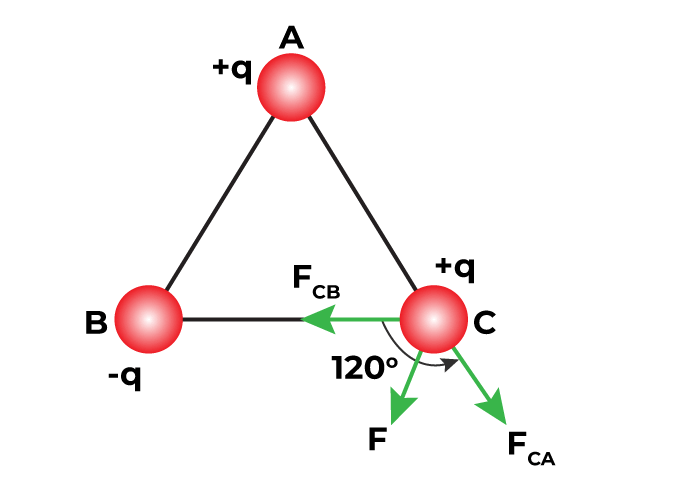
Figure 9: Coulomb's Law
Charles Coulomb's experiments were very helpful for understanding electrostatic force. He discovered that the force between two electric charges decreases quickly as the distance between them increases. Basically, as you move the charges further apart, the force between them gets much weaker. This idea is similar to Newton's Law of Gravitation, which says that the gravitational force between two masses also decreases as the distance between them increases.
In Coulomb's Law, the main idea is that the force between charges becomes weaker if you increase the distance and stronger if you decrease the distance. This behavior is like how gravitational force works, but instead of dealing with masses and gravity, Coulomb's Law deals with electric charges.
This knowledge is very helpful for explaining many electrical things. For example, if you double the distance between two charged objects, the force pulling or pushing them together becomes much weaker. On the other hand, bringing the objects closer together makes the force much stronger.
Coulomb's Law has many uses in science and engineering. It helps in designing electronic parts like capacitors, understanding how atoms join together, and predicting how static electricity behaves in different situations. Coulomb's work laid the foundation for modern ideas of electromagnetism and remains very significant to the study of physics and electrical engineering.
Voltage and Amperage
Electric current is basically the flow of electrons through a conductor. This flow has two main properties: voltage and amperage. Voltage, also called electric potential, is the force that pushes electrons through a circuit, similar to water pressure in a pipe. Amperage, or current flow, is the number of electrons moving through the circuit, like the amount of water flowing through the pipe.
In everyday household electrical systems, the standard voltage is usually around 120 volts. Different appliances use varying amounts of amperage based on their power needs. For example, a light bulb uses a small amount of current, while a large appliance like an oven or a washing machine uses much more.
Electric power, which is the rate at which electrical energy is used or produced, is calculated by multiplying voltage and amperage (P = V × I). This means that an appliance running at 120 volts and using 10 amperes of current uses 1,200 watts of power.
Static electricity, on the other hand, can create very high voltages but usually involves very low amperage. This is why the shocks we get from static electricity can be surprising but are generally harmless. The high voltage can easily push electrons through the air, causing a spark, but the low amperage means that the total energy involved is very small.
Electrostatics in Daily Life
Static electricity is something we often encounter in daily life. When you walk across a carpet or take off a hat, you might get a shock when you touch a metal object. This happens because your body collects an electric charge.
This charge builds up when electrons move from one thing to another. For example, as you walk on a carpet, electrons move from the carpet to your shoes, making your body negatively charged. When you touch a metal object, which easily allows electricity to flow, the extra electrons in your body quickly move to the metal, causing a small electric shock.
This effect is stronger when you are separated from the ground by materials that do not allow electricity to flow easily, such as rubber-soled shoes. These materials stop the electrons from easily escaping into the ground, causing the charge to build up on your body. So, the shock you feel is the quick movement of electrons from your body to something that can conduct electricity.
Conclusion
The exploration of static electricity, from early observations to significant scientific discoveries, shows how our understanding of electrical phenomena has evolved. Curiosity about why materials attract and repel each other led to groundbreaking theories by pioneers like Charles Dufay and Benjamin Franklin. They discovered that the movement of electrons is the basis for electric charge. The creation of electrostatic machines and the Leyden jar allowed scientists to generate and study large static charges. This work culminated in Franklin's demonstration that lightning is an electrical discharge. Charles Coulomb further established the principles of static electricity by formulating the laws of electric force. These discoveries have not only advanced theoretical knowledge but also led to practical applications such as xerographic printing, electrostatic air filters, and the Van de Graaff generator. Understanding static electricity plays a key role in everyday experiences and scientific endeavors, highlighting its role in physics and technology.
Frequently Asked Questions [FAQ]
1. How do I stop getting shocked by everything I touch?
To stop getting shocked by everything you touch, increase the moisture in your environment by using a humidifier. Wearing shoes with leather soles instead of rubber can help, as leather does not create as much static electricity. Also, before touching anything else, try touching a metal object to discharge any static buildup from your body.
2. How to ground yourself to avoid static shock?
To prevent static shock, frequently touch a grounded metal object. Using anti-static wristbands or grounding mats can also help remove static electricity from your body, reducing the chance of getting shocked.
3. What triggers static?
Static electricity happens when materials rub against each other. Simple actions like walking on a carpet with socks, taking off synthetic fabric clothes, or even sitting on certain types of furniture can cause electrons to move from one material to another. This movement creates an imbalance, which results in static electricity.
4. Why do I get electric shocks when I touch something?
You get electric shocks when you touch something because your body has built up a static charge. When you touch a conductive object, like metal or another person, the built-up charge quickly flows out of your body, resulting in a shock.
5. How to avoid static electricity on PC?
To avoid static electricity on your PC, use an anti-static wrist strap while working inside the computer. Make sure your PC is placed on a grounded surface, and avoid working in dry environments. You can also use anti-static mats or sprays to reduce static buildup around your work area.
About us
ALLELCO LIMITED
Read more
Quick inquiry
Please send an inquiry, we will respond immediately.
Achieving Peak Performance with the Maximum Power Transfer Theorem
on June 20th
Understanding the Power of the S-R Latch: A Gateway to Modern Electronics
on June 19th
Popular Posts
-
What is GND in the circuit?
on January 1th 2937
-

RJ-45 Connector Guide: RJ-45 Connector Color Codes, Wiring Schemes, R-J45 Applications, RJ-45 Datasheets
on January 1th 2499
-

Fiber Connector Types: SC Vs LC And LC Vs MTP
on January 1th 2089
-
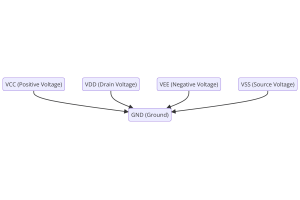
Understanding Power Supply Voltages in Electronics VCC, VDD, VEE, VSS, and GND
on November 9th 1890
-

Comparison Between DB9 and RS232
on January 1th 1761
-

What Is An LR44 Battery?
Electricity, that ubiquitous force, quietly permeates every aspect of our daily lives, from trivial gadgets to life-threatening medical equipment, it plays a silent role. However, truly grasping this energy, especially how to store and efficiently output it, is no easy task. It is against this background that this article will focus on a type of coin cell battery that may seem insignificant on the...on January 1th 1712
-
Understanding the Fundamentals:Inductance Resistance, andCapacitance
In the intricate dance of electrical engineering, a trio of fundamental elements takes center stage: inductance, resistance, and capacitance. Each bears unique traits that dictate the dynamic rhythms of electronic circuits. Here, we embark on a journey to decipher the complexities of these components, to uncover their distinct roles and practical uses within the vast electrical orchestra. Inductan...on January 1th 1652
-

CR2430 Battery Comprehensive Guide: Specifications, Applications and Comparison to CR2032 Batteries
What is CR2430 battery ?Benefits of CR2430 BatteriesNormCR2430 Battery ApplicationsCR2430 EquivalentCR2430 VS CR2032Battery CR2430 SizeWhat to look for when buying the CR2430 and equivalentsData Sheet PDFFrequently Asked Questions Batteries are the heart of small electronic devices. Among the many types available, coin cells play a crucial role, commonly found in calculators, remote controls, and ...on January 1th 1549
-
What Is RF and Why Do We Use It?
Radio Frequency (RF) technology is a key part of modern wireless communication, enabling data transmission over long distances without physical connections. This article delves into the basics of RF, explaining how electromagnetic radiation (EMR) makes RF communication possible. We will explore the principles of EMR, the creation and control of RF signals, and their wide-ranging uses. The article ...on January 1th 1537
-

CR2450 vs CR2032: Can The Battery Be Used Instead?
Lithium manganese batteries do have some similarities with other lithium batteries. High energy density and long service life are the characteristics they have in common. This kind of battery has won the trust and favor of many consumers because of its unique safety. Expensive tech gadgets? Small appliances in our homes? Look around and you'll see them everywhere. Among these many lithium-manganes...on January 1th 1507















































